 The interoperability of healthcare information systems is one of the most important challenges facing both users and suppliers of healthcare solutions. To meet these challenges, IHE International is introducing the IHE Conformity Assessment Programme.
The interoperability of healthcare information systems is one of the most important challenges facing both users and suppliers of healthcare solutions. To meet these challenges, IHE International is introducing the IHE Conformity Assessment Programme.
The IHE Conformity Assessment testing is based on an ISO/IEC 17025 quality system in accordance with the IHE Conformity Assessment Scheme published by IHE. A specific set of IHE Profiles used for sharing health records is available for testing in accordance with requests from projects users and the industry (to learn more, please visit the link at the bottom of the page).
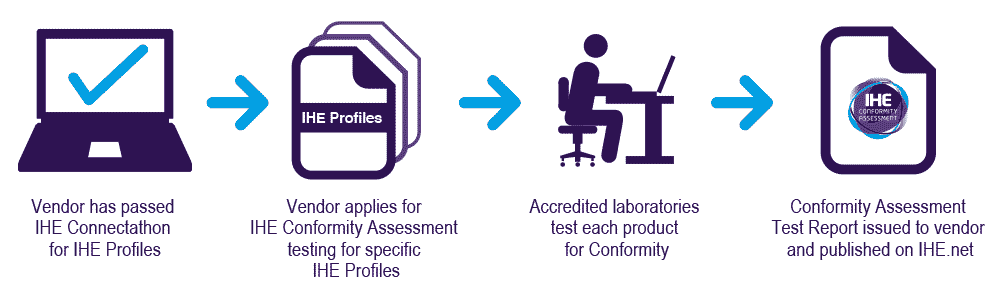
Products submitted must be either market-released products or expected to be released within six months after the Conformity Assessment test session. To engage in Conformity Assessment testing, the vendor must have passed the IHE Connectathon tests within the prior two years for the appropriate IHE Profiles targeted for Conformity Assessment. The accredited testing laboratory, authorised by IHE International, will deliver the Conformity Assessment Report that is published on the IHE International website after successful completion of testing. See:
Value of Conformity Assessment
The accurate, timely and secure exchange of medical records requires unique technical expertise and competencies often beyond the experience of individual vendor or user deployment teams.
Medical information details often provide crucial facts needed for optimal healthcare, whether within a hospital, across regional health IT projects, within national networks, or from a hospital to the patient at home. It is critical that vendors and users work together, along with regulatory authorities and standards bodies, to ensure that products, systems and solutions interoperate together to bring quality solutions to the market that perform as they should and result in the best quality patient care.
To reduce costs, delays and other risks of incorrect, inappropriate and inadequate product purchases of many products, users have come to depend on trusted, independent third-party testing, which is often called “Conformity Assessment”.
Benefits > End Users
- Large eHealth projects reduce their testing and integration efforts by specifying and procuring products that have been conformity-assessed.
- To the end user it gives confidence that a current/potential supplier has independent proof of the interoperability of their products.
- Rely on an accredited testing laboratory to validate products before they are installed in an organisation or facility, reducing risks and deployment costs.
- Improved patient outcome through better and more consistent product quality.
Benefits > Suppliers
- Increase the interoperability readiness for systems and solutions.
- Gain global market credibility by distinguishing the company and its products. For a listing of companies and products, please visit: http://conformity.ihe.net/summary-reports.
- Be recognised internationally and accepted for “shortlisting” (i.e. pre-qualified for purchasing programmes) by being engaged in the quality process governed by the IHE Conformity Assessment Programme.
- Benefit from a wealth of IHE Profiles and increase an organisation’s capabilities.



